Projects



Nanoplastic particles: The adverse effects of nanoplastic particles in zebrafish: short- and long term consequences
In this project zebrafish are exposed to different concentrations of a 0,5 µm polystyrene nanoplastic particle. For 110 days from the moment of fertilization the fish were exposed and short- and longterm adverse effects of the particle was analyzed. For the short term effects 5 and 12 days old larvae were sampled for gene expression and metabolomic analyses. Data are still being analyzed. For the long term effects reproduction, length and weight (Fulton’s condition factor) and the metabolome were measured and analyzed and some of these data are still in process. Preliminary results include tendencies towards a lower Fulton’s condition factor when exposed to the particle and dysbiosis of the microbiome.
Journal of Hazardous Materials
Staphylococcus aureus represents a growing challenge for human health, particularly methicillin-resistant (MRSA) and vancomycin-intermediate strains (VISA), which are increasingly difficult to treat. Improved in vivo models are needed to study host–pathogen interactions and support the development of new therapeutic strategies. In this project, a zebrafish larval immersion infection model is established to study S. aureus infections. To mimic natural wound infections, larvae undergo a controlled tail amputation prior to bacterial exposure. The model allows infection of large numbers of larvae simultaneously and enables real-time visualization of innate immune responses due to the transparency of zebrafish larvae.
The project makes use of transgenic zebrafish lines with fluorescently labelled innate immune cells, enabling visualization of neutrophil and macrophage responses during infection. Using this system, differences in virulence between MRSA and VISA strains are investigated, with particular focus on how mutations affecting the bacterial cell wall influence host immune responses. The model provides a fast, scalable, and ethically favorable platform for studying antibiotic-resistant bacterial infections in vivo.
Ms Student Ditte Goldschmidt Jepsen; supervisor Louise von Gersdorff Jørgensen.
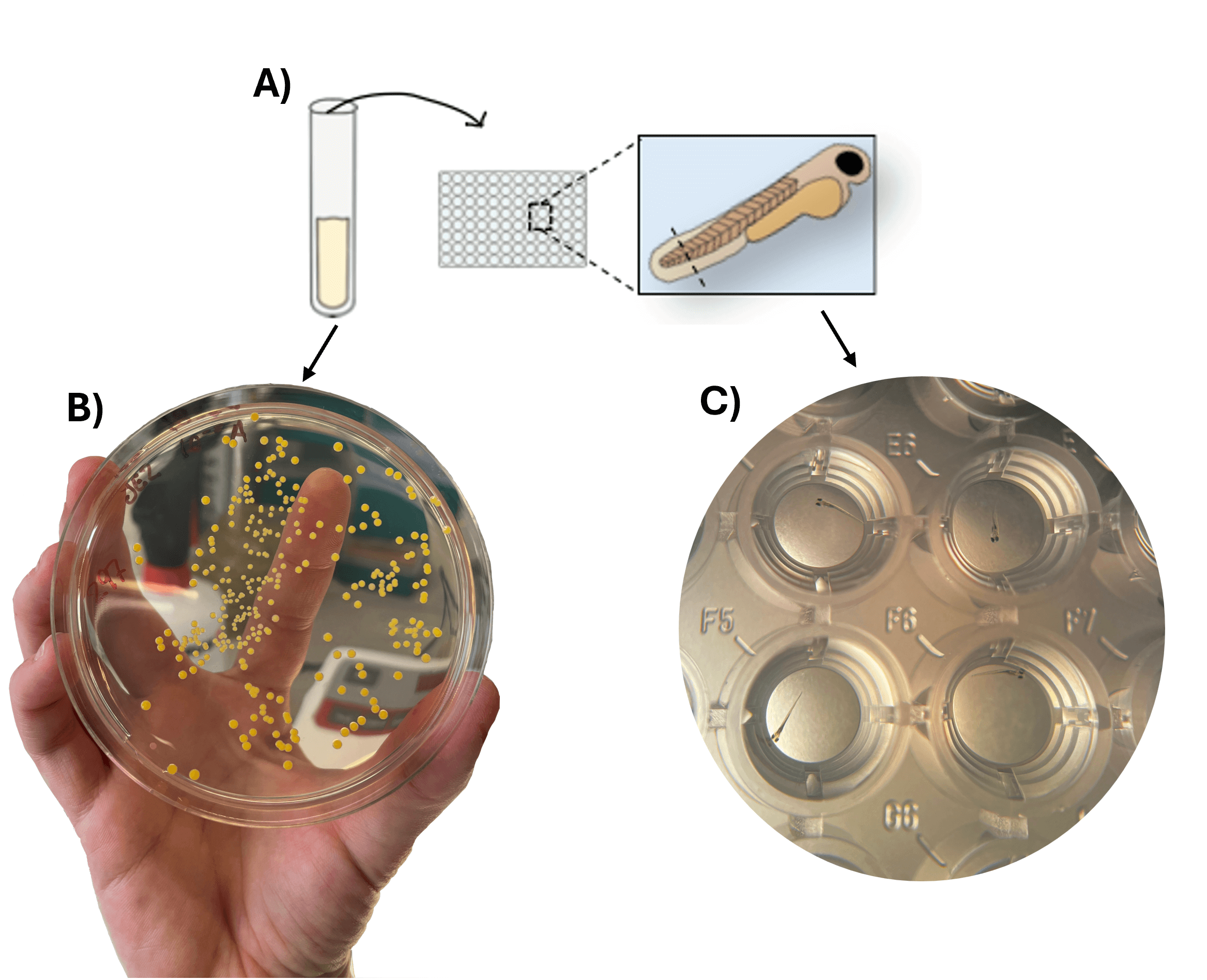
Figur tekst: Tail-cut immersion infection model in zebrafish larvae, showing (A) a schematic overview of the infection method, (B) Staphylococcus aureus grown on agar plates, and (C) larvae housed in a 96-well plate during infection.
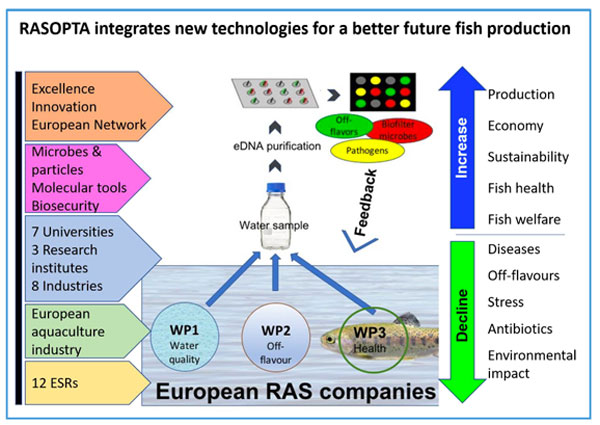
RASOPTA - Safeguarding future production of fish in aquaculture systems with water recirculation
This cross European project, coordinated by the University of Copenhagen is focused on water quality, fish health and off-flavours in European aquaculture. In the project a Fluidigm chip will be developed quantifying a wide spectrum of microorganisms and pathogens in the water using environmental DNA (eDNA) from fish farm water to be able to surveille for potential disease outbreaks. We will use zebrafish as a model organism for carp and relate the amount of eDNA in the water to the state of disease in the zebrafish and the load of pathogens in the fish and in the water.

ProFishence – developing high efficient feed for production fish using zebrafish as a screening model
In collaboration with Chr. Hansen and Aller Aqua we will develop feed containing high efficient probiotic bacteria. The aim is to apply the feed for production fish but in order to select suitable organisms we will use zebrafish as a screening model. Fluorescent potential probiotic bacteria will be selected and fed to zebrafish. Using real-time imaging, we will evaluate the different probionts to select the ones that are able to colonize the gut and aid in fish health. Selected probionts will subsequently be fed and evaluated in rainbow trout.
Aquaculture is the fastest growing sector producing animal protein for human consumption. Further production increases are however limited by fish diseases, and innovative solutions to solve this problem are warranted. The single-celled freshwater fish parasite Ichthyophthirius multifiliis (Ich) causes severe production losses worldwide, since most commercial fish species, including rainbow trout (RT), are very susceptible. In contrast, zebrafish are resistant to infection by Ich.
In a new approach to address this problem, candidate resistance genes will be identified by comparing transcriptomes from zebrafish and RT after exposure to the parasite, and tested by introducing susceptibility using CRISPR/Cas9 knockouts.
Once resistance genes have been identified, it will be possible to use either selective breeding or genetic modification to obtain production fish with strong expression of the resistance gene products. Other diseases could furthermore be targeted and production improved.
PhD Student Heidi Mathiessen; supervisor Louise von Gersdorff Jørgensen
This is a Master Thesis Project of Carlota Marola Fernandez Gonzalez in association with Kristian Syberg and Ole Vang from Roskilde University. The study includes in vivo and in vitro experiments. The in vivo study comprises the embryogenic and early larval stages of zebrafish (Danio rerio) and the in vitro study involves the zebrafish fibroblast-like cell line ZF4 established from 1-day-old zebrafish embryos. Nanoplastic concentrations remain the same during the whole investigation, except for the FET (Fish Embryo Toxicity Test). The concentrations are chosen according to what is regarded environmentally realistic so far - 15 µg/l [1]
Zebrafish embryos are exposed to different concentrations of nanoplastics according to the FET (OECD 236). This acute test uses short-term (96 hours) exposure to determine the concentration that is lethal to 50% of the zebrafish embryos (LC50) as an indicator of acute fish toxicity. We include the following death indicators: coagulation of the embryo, lack of somite formation (a somite is an early stage division of a body part of an embryo), lack of heartbeat, malformations and immobilization. All these damages are easily detectable using light microscopy. We also investigated the regenerative capacity of zebrafish tailfin during the exposure to nanoplastics, concluding that the complete regeneration occurs during 96h.
Apart from evaluating tissue regrowth (nm since cut per day post amputation -nm/dpa), we also counted the number of macrophages and neutrophils within the new tissue.
In vitro studies include counting the cell number, density and cellular metabolism of ZF4 cell line after different nanoplastic exposure durations. The Hoechst staining method is used to estimate cell density and the MTT colorimetric assay to measure cell metabolic activity. All these investigations are coupled with confocal microscopy to localize nanoplastic aggregates inside the cells.
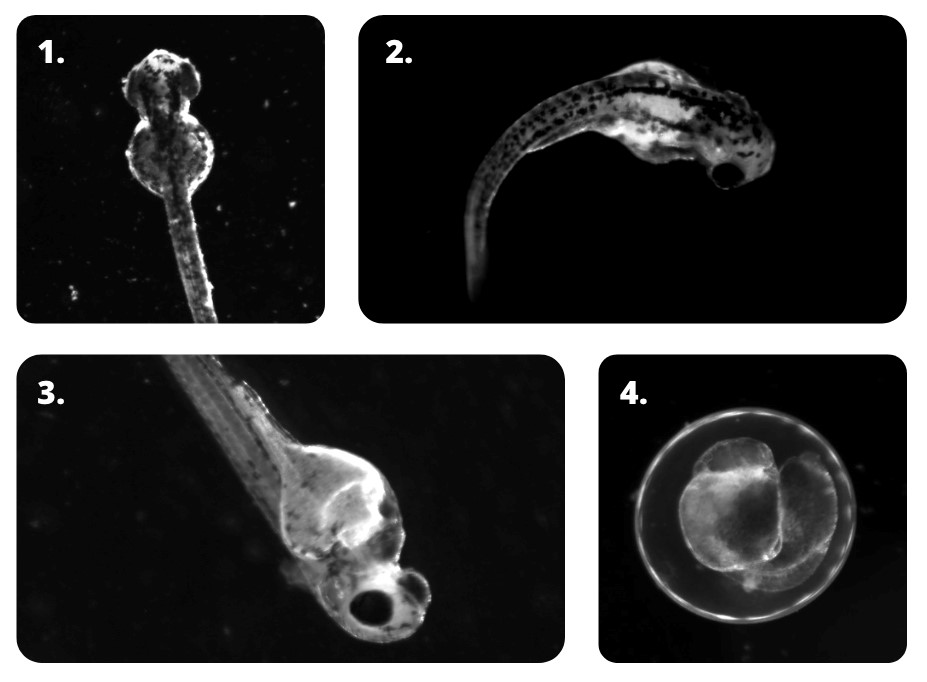 Lethality indicators after nanoplastic exposure and positive controls. 1.Pericardium edema 2. Scoliosis 3. Pericardium edema 4. Embryo malformation.
Lethality indicators after nanoplastic exposure and positive controls. 1.Pericardium edema 2. Scoliosis 3. Pericardium edema 4. Embryo malformation.
[1] Al-Sid-Cheikh et al. Uptake, Whole-Body Distribution, and Depuration of Nanoplastics by the Scallop Pecten maximus at Environmentally Realistic Concentrations. Environ Sci Technol. 2018 Dec 18;52(24):14480-14486. doi: 10.1021/acs.est.8b05266
